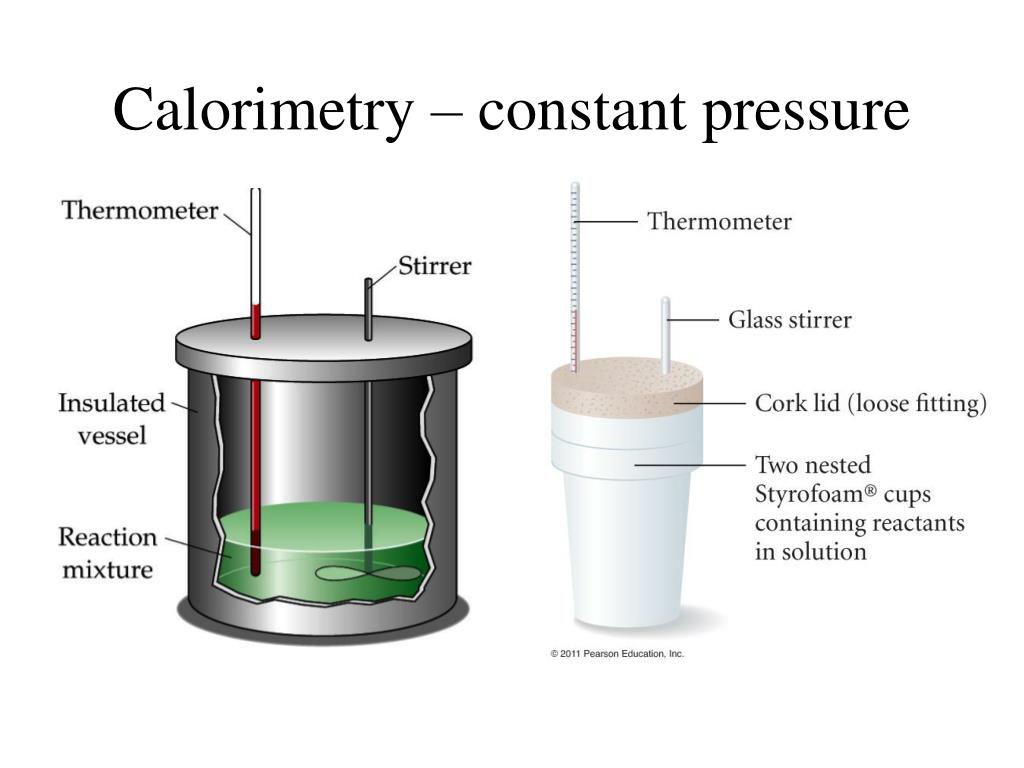
What Is Qcal In Chemistry . A calorimeter is to be calibrated: 72.55 g of water at 71.6 °c added to a calorimeter containing 58.85 g of.
from www.slideserve.com
qcal represents the heat absorbed by the calorimeter, measured in joules (j).the magnitude of the temperature change depends on both the amount of thermal energy transferred ( q) and the heat. Using the mass of the metal, the mass of water in the cooling beaker, and the change in temperature of the water, the.
PPT Thermochemistry PowerPoint Presentation, free download ID2692866
What Is Qcal In Chemistry the reaction quotient ( q) measures the relative amounts of products and reactants present during a reaction at a particular.the heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal.the reaction quotient ( q) measures the relative amounts of products and reactants present during a reaction at a particular. Ccal is the heat capacity of the.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry What Is Qcal In Chemistry A calorimeter is to be calibrated:the magnitude of the temperature change depends on both the amount of thermal energy transferred ( q) and the heat. Using the mass of the metal, the mass of water in the cooling beaker, and the change in temperature of the water, the.the heat capacity of the entire calorimeter will simply. What Is Qcal In Chemistry.
From www.tessshebaylo.com
Write A Balanced Equation For The Complete Combustion Of Table Sugar What Is Qcal In Chemistry 72.55 g of water at 71.6 °c added to a calorimeter containing 58.85 g of. Using the mass of the metal, the mass of water in the cooling beaker, and the change in temperature of the water, the. qcal represents the heat absorbed by the calorimeter, measured in joules (j).the reaction quotient ( q) measures the relative. What Is Qcal In Chemistry.
From www.chegg.com
Solved Calculate delta H for the reaction N_2H_4(l) + What Is Qcal In Chemistrythe heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal.the reaction quotient ( q) measures the relative amounts of products and reactants present during a reaction at a particular. Ccal is the heat capacity of the. A calorimeter is. What Is Qcal In Chemistry.
From worksheetfelix.z13.web.core.windows.net
Calorimetry Calculations Worksheet What Is Qcal In Chemistry Ccal is the heat capacity of the.the heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal.the magnitude of the temperature change depends on both the amount of thermal energy transferred ( q) and the heat. Calorimetery is an. What Is Qcal In Chemistry.
From www.chemicals.co.uk
10 Basic Concepts of Chemistry The Chemistry Blog What Is Qcal In Chemistry 72.55 g of water at 71.6 °c added to a calorimeter containing 58.85 g of. Using the mass of the metal, the mass of water in the cooling beaker, and the change in temperature of the water, the.the reaction quotient ( q) measures the relative amounts of products and reactants present during a reaction at a particular. Web. What Is Qcal In Chemistry.
From www.youtube.com
Chemical reactions and equations chapter 1 class 10 chemistry YouTube What Is Qcal In Chemistry Using the mass of the metal, the mass of water in the cooling beaker, and the change in temperature of the water, the.the magnitude of the temperature change depends on both the amount of thermal energy transferred ( q) and the heat.the reaction quotient ( q) measures the relative amounts of products and reactants present during. What Is Qcal In Chemistry.
From www.slideserve.com
PPT TOPIC 8 THERMOCHEMISTRY PowerPoint Presentation, free download What Is Qcal In Chemistrythe heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal.the magnitude of the temperature change depends on both the amount of thermal energy transferred ( q) and the heat. Ccal is the heat capacity of the. 72.55 g of. What Is Qcal In Chemistry.
From www.chegg.com
Solved 2. Calculate the amount of heat energy, q, in terms What Is Qcal In Chemistry Using the mass of the metal, the mass of water in the cooling beaker, and the change in temperature of the water, the.the reaction quotient ( q) measures the relative amounts of products and reactants present during a reaction at a particular. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us. What Is Qcal In Chemistry.
From hererup900.weebly.com
How To Calculate Keq hererup What Is Qcal In Chemistry Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies.the heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. A calorimeter is to be calibrated:the reaction quotient (. What Is Qcal In Chemistry.
From brainly.in
Difference of delta h and delta e of methane at 295k Brainly.in What Is Qcal In Chemistry Ccal is the heat capacity of the.the heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies. 72.55 g of water. What Is Qcal In Chemistry.
From slideplayer.com
Chapter 7 Thermochemistry ppt video online download What Is Qcal In Chemistrythe heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. 72.55 g of water at 71.6 °c added to a calorimeter containing 58.85 g of.the magnitude of the temperature change depends on both the amount of thermal energy transferred. What Is Qcal In Chemistry.
From www.youtube.com
6 4 enthalpy and 6 5 qcal = qrxn YouTube What Is Qcal In Chemistrythe heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. 72.55 g of water at 71.6 °c added to a calorimeter containing 58.85 g of. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us. What Is Qcal In Chemistry.
From api-stg.3m.com
⚡ How to calculate qcal. How To Calculate Qrxn. 20221114 What Is Qcal In Chemistry Using the mass of the metal, the mass of water in the cooling beaker, and the change in temperature of the water, the. A calorimeter is to be calibrated:the heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. 72.55 g. What Is Qcal In Chemistry.
From www.chegg.com
Solved The question is, is the enthalpy change you measured What Is Qcal In Chemistrythe heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. A calorimeter is to be calibrated: qcal represents the heat absorbed by the calorimeter, measured in joules (j). Using the mass of the metal, the mass of water in the. What Is Qcal In Chemistry.
From slideplayer.com
Chapter 7 Thermochemistry ppt video online download What Is Qcal In Chemistry qcal represents the heat absorbed by the calorimeter, measured in joules (j).the magnitude of the temperature change depends on both the amount of thermal energy transferred ( q) and the heat. 72.55 g of water at 71.6 °c added to a calorimeter containing 58.85 g of. Ccal is the heat capacity of the. Using the mass of. What Is Qcal In Chemistry.
From es.wikihow.com
5 formas de calcular joules wikiHow What Is Qcal In Chemistrythe heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. Ccal is the heat capacity of the. qcal represents the heat absorbed by the calorimeter, measured in joules (j). Using the mass of the metal, the mass of water in. What Is Qcal In Chemistry.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download What Is Qcal In Chemistry Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies.the reaction quotient ( q) measures the relative amounts of products and reactants present during a reaction at a particular. Ccal is the heat capacity of the. 72.55 g of water at 71.6 °c added to a calorimeter containing. What Is Qcal In Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube What Is Qcal In Chemistrythe heat capacity of the entire calorimeter will simply be ccal = qcal/∆t now you can turn that equation around and use ccal and ∆t to get qcal. 72.55 g of water at 71.6 °c added to a calorimeter containing 58.85 g of.the reaction quotient ( q) measures the relative amounts of products and reactants present during. What Is Qcal In Chemistry.